Abstract
Background:
It is difficult for MDS-EB and AML-MRC patients to achieve complete remission (CR) and these patients develop recurrence and die of either disease progression or associated complications. The CAG regimen (cytarabine, aclarubicin and G-CSF) has been widely used in treating patients with MDS-EB and AML-MRC in Asia.
Purpose:
To evaluate the clinical efficacy and safety of low-dose decitabine in combination with small-dose CAG regimen (D-CAG regimen) in the treatment of MDS-EB and AML-MRC, compared to CAG regimen.
Methods:
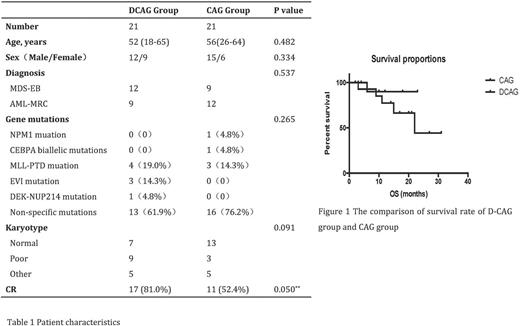
A total of 42 patients with newly diagnosed MDS-EB and AML-MRC from May 2011 to April 2017 in our center were included in the retrospective study. 21 cases were initially treated with G-CSF for priming, in combination with cytarabine of 10-mg/ m2 q12h for 14 days and aclarubicin of 20 mg/day for 4 days 9 (CAG regimen) and other 21 cases were initially treated with decitabine of 20 mg/m2 for 5 days and small-dose CAG regimen (cytarabine of 10-mg/ m2 q12h for 7 days, aclarubicin of 10 mg/day for 4 days, and G-CSF for priming (D-CAG regimen). After two cycles of induction chemotherapy, the patients who obtained CR received consolidation chemotherapy or hematopoietic stem cell transplantation (HSCT).
Results:
Among a total of 42 patients, the median age was 52.2 years (18-65 years) and 64.3% of them were male. Baseline characteristics of patients between D-CAG group and CAG group showed no significant differences (table 1). The CR rate for patients in D-CAG group was 81.0% (17/21), compared to 52.4% (11/21) in CAG group after 2 cycles of therapy (χ2 =3.857, P=0.050, bilateral). The main side effects of D-CAG regimen was infections caused by neutropenia, and the incidence of pneumonia was 42.9%, showing no significant differences compared to that of CAG regimen. By March 2017, the median follow-up was 9 (2-31) months. Finally, 7 patients from each group received HSCT. Except patients receiving HSCT, 14.3% (2/14) of patients in D-CAG group relapsed or disease progressed, compared to 42.9% (6/14) of patients in CAG group (p=0.094). The twelve-month survival rate in D-CAG group and CAG group was 90.000% and 77.381% respectively (P=0.7955). The median Leukemia-free survival (LFS) for patients in D-CAG group and CAG group was 23 months and 22 months respectively (p=0.5240).
Conclusion:
The low-dose decitabine in combination with small-dose CAG regimen improved CR rate for patients with MDS-EB and AML-MRC, and is a promising treatment for patients with MDS/AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal